My name is Sheldon Margulies. I am a retired board-certified neurologist who has taught over 2500 medical students and residents at the University of Alabama and University of Maryland. I also have a law degree from the University of Baltimore.
In 2005, based on my understanding of how the brain learns and applying what I’d learned teaching medical students and residents, I turned my attention to what I believe was, and still is, a critical weakness in American education, namely, the teaching of science in grammar school, middle school, and high school.
The Problem
For decades, science education of middle and high school students in the United States has been struggling to improve. Efforts to improve the situation have failed. Between 2009 and 2019, the National Assessment of Educational Progress, the “nation’s report card,” reported no improvement despite the creation in 2013 of the Next Generation Science Standards which have now been adopted by 20 states.
The Next Generation Science Standards
The Next Generation Science Standards (NGSS) were introduced in 2013 to help improve science education. NGSS advocates that, instead of simply memorizing facts, students master scientific concepts by confronting problems with questions, developing models, carrying out experiments, analyzing the experimental data, and drawing conclusions from the data. In the process, students will begin to see patterns such as cause and effect, proportionality, systems, energy and matter, structure and function, and stability and change. In short, students learn by confronting puzzling phenomena and problem situations, by asking questions, creating models, performing experiments, and analyzing experimental data.
Despite having been around for upwards of 13 years, the adoption of NGSS in many states has failed to improve national science test scores. In fact, no teaching methodology or science textbook has ever been shown to be more effective than any other teaching methodology or textbook.
NGSS does not dictate that science be taught in any particular way. The goal is to change science education from a teacher explaining things and from students memorizing facts and details to an approach where teachers teach fewer but more usable scientific ideas, and also more engineering and technology. The goal is to get students to view a phenomenon, develop their own explanations (with guidance from the teacher), and then plan and perform experiments to confirm or refute their explanations.
Getting students to view a phenomenon, develop their own explanations for how and why it occurred, and plan and perform experiments to confirm or refute their explanations are all worthwhile goals. Where I think more emphasis is needed is in providing students with more knowledge to explain puzzling phenomena. That knowledge is in the atomic structure of matter, because all forces emerge from the atom — gravitational forces, electrical forces, magnetic forces, electromagnetic forces, and nuclear forces.
Understanding how atomic structures create and control everyday phenomena is essential if students are expected to manipulate the structure of matter and meet the goals of The Next Generation Science Standards to incorporate more engineering into STEM education.
The Big Pictures
Teaching the atomic structure of matter, and how atomic structures create the 5 forces of nature, begins in chemistry. I’ve found that students do best when first provided with “the big picture of chemistry,” the small central core of knowledge from which everything else in chemistry stems.
For chemistry, the big picture is that there are about 100 different types of atoms in the world, but they bond to each other to form molecules in only one of four ways. Each type of bonding produces molecules with characteristic properties: they may form a gas, liquid, or solid; they may or may not dissolve in water; they might be shiny or dull; they may form metals that bend or crystals that crack; they may or may not react with other molecules, and so on. The rest of chemistry consists of different ways to measure those properties and learning how to change the properties.
The big picture in biology is imagining earth three and a half billion years ago, before life began, and thinking of what it would take for life to begin. What does every living thing need? Life, of course, would need a confined place to tinker with molecules, specifically, a tiny cell with waterproof walls, because without a confined space, any important molecule would be washed away in the ocean. Cells would need a way to take in energy, such as the energy in sunlight, ultraviolet light, heat, wind energy, lightning, or even the energy stored in chemical bonds.
Living things would need a way to manipulate the energy and regulate the entry of nutrients into the cell so the cell could grow and develop more efficient ways to obtain energy and nutrients. Living things certainly need a way to reproduce and discharge waste, and since chemical reactions taking place inside the cell are sensitive to pH, temperature, and concentration, living things also need a way to keep their internal
environment stable. And if the environment changes, they need a way to adapt to changes in the external environment. Biology is the study of how all this is done.
The big picture in physics is the study of forces — mechanical forces, electrical forces, chemical forces, magnetic forces, electromagnetic forces, and nuclear forces.
Physics’ big picture is the fundamental forces of nature, all stemming from the atom — gravitational forces, electrical forces, magnetic forces, electromagnetic forces, and nuclear forces — how they’re created, how we measure them, and how we harness and manipulate them.
Audiovisual instruction
I believe that an audiovisual approach, where students see and hear what’s being taught instead of reading it is more effective, because it allows the brain to store what’s being taught in both its visual and auditory regions. By providing students with evolving images, students see what’s being said and understand the material sooner. Here is evidence to support my conclusion.
Dunbar High School
In 2005, I began creating audiovisual-based science curricula in chemistry and biology. In 2007, after discussing my concerns and my teaching methodology with Dr. John Ulatowski, Chairman of the Department of Anesthesiology at Johns Hopkins School of Medicine, Dr. Ulatowski asked me to help teach biology at Dunbar High School, a major inner-city high school in Baltimore with which Johns Hopkins had a mentoring relationship. The problem at Dunbar High School was that the graduation rate had fallen to only 40 percent of students, because only 40 percent of the students were able to pass the High School Assessment (HSA) exams in biology, a requirement for graduation.
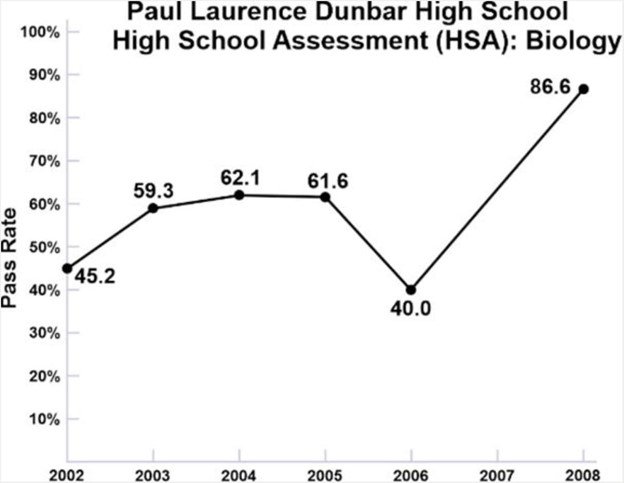
This chart depicts the pass rate for the HSA exams prior to my intervention. (There was no data for 2007 for reasons unknown.)
Biology at Dunbar High School was taught 5 days a week. I agreed to teach my visual-based biology curriculum for 1 hour, 1 day a week, to 4 sections of the junior class, 97 students total, from September 2007 to June 2008. That academic year, 87% of the junior class passed the HSA biology exams and graduated high school.
Why the improvement?
There are a number of reasons for the dramatic improvement in the Dunbar students’ performance. The primary reason is that I made biology easy to understand and thus easy to remember. I did this by first providing students with an overview, or “big picture,” of biology and then explaining each topic in the big picture with narrated, evolving images instead of simply written text.
By explaining each of these topics with narrated images, students were able to see what was being said as it was said. And by allowing the illustrations to develop in complexity, students were able to follow my accompanying verbal explanations of the developing images and see how biologic structures and processes fit together. These narrated images relieved students from having to switch their attention back and forth between a textbook’s text and all the accompanying illustrations, charts, graphs, and sidebars.
Teaching Methodology
Having taught over 2500 medical students and residents in my career, I have discovered that it is impossible to underestimate what students know. Accordingly, I reassure students that I’m not there to find out how smart they are. I’m there to impart a skill they can use in their own lives, meaning that I treat students as my apprentices with little if any emphasis of grades.
I know that high school students come to science with their trigger cocked, ready to throw up their hands at the slightest moment of confusion and frustration. Students are all too ready to announce that science is just too hard and is really only for the smart kids in the class.
I reassure students that everyone suffers from the fear that their friends, acquaintances, and teachers are about to discover how inept they are and hold them up for public humiliation. I reassure students that I know they know nothing about anything. That’s why I’m there — to help them become more than they ever thought they could be.
In my experience, audiovisual presentations make chemistry, biology, and physics easier to understand and remember, for a number of reasons. First off, many students are not good readers. Second, audiovisual presentations allow students to simultaneously see and hear what’s being explained instead of having to dart their attention back and forth between written text and static images. And from a neurologic standpoint, audiovisual presentations allow the subject matter to be stored in visual and auditory areas of the brain, making
for easier access and retrieval later.
Also, audiovisual presentations allow for a “catch our breath” slide midway through each lesson. This breather slide summarizes what’s just been taught and prevents students from being overwhelmed by too many facts at one time.
Following each lesson is a review lesson that refreshes the student’s memory by asking questions based on the lesson and then providing answers. Following that is a multiple-choice test for each lesson, with hints, followed by explanations of the answers for their review.
Another reason students at Dunbar did so well is that I made use of positive emotions. Emotions are essential to laying down new memories. For something to be remembered, it must first be attached to an emotion. This takes place in the brain’s hippocampus, one of the major emotional centers of the limbic system of the brain. For something to be remembered, the hippocampus attaches an emotion to the memory trace. While any emotion will do, learning benefits most from positive emotions, like wonder, excitement, surprise, and the joy of success. It thus behooves us to avoid any negative emotions associated with anxiety, fear, embarassment, humiliation, or anger.
Still another reason for their success was that I made the material relevant to their lives. To give an example, after explaining diffusion across membranes, I presented a true case of a Baltimore woman who, to win money to buy Christmas presents, entered a radio contest. Whoever drank the most water in an hour would win. She drank a gallon or more and lapsed into a coma. I asked the class to pretend they were the emergency room doctor and explain why she lapsed into a coma and how they would treat her. The majority of the class
knew that water had diffused into her brain, causing brain swelling, but only about a third of the class correctly suggested giving her hypertonic saline to allow water to diffuse out her brain. Science was now something practical and useful.
What I am trying to do with these curricula is omit the phrase “I don’t get it” from students’ lexicon. Many kids come to science believing in their hearts that science is just too difficult and is really only for the smart kids in the class. They arrive with their trigger cocked, ready to throw up their hands at the slightest difficulty and announce that “This is just too hard. I can’t do it.”

To counter the misconception that science is only for the “smart kids” in the class, I don’t teach science, I explain it. I don’t want students to just learn about chemistry, biology, and physics, I want them to use them. Students need to see that science makes sense, and that it explains things they’ve encountered or could encounter, such as how soap cuts grease, how a pressure cooker works, the chemistry behind using the Maillard reaction to make barbecued steaks more flavorful, the meaning of relative humidity, the neurologic risks that lead in drinking water poses to infants and toddlers, and so on.
It’s worth emphasizing that audiovisual curricula allow science to be presented in a clear, concise, and understandable way, and at the same time be available via the Internet to students living in remote areas of the country and beyond our borders. Audiovisual curricula also allow us to adapt to the way students now communicate. Given how attached kids are to their iPhones, iPads, and computers, we need to shift science education away from textbooks to a more audiovisual approach.
Grades
I’m not a fan of grades. While grades do reflect how well a student is doing compared to everyone else in the class, they don’t reflect how much the student has accomplished since the course started, which is equally important to that student, the parents, and the teacher. The ones most interested in grades are colleges trying to decide whom to admit.
While grades do help motivate students, they also add considerable anxiety, which only distracts from learning and adds unnecessary stress for students trying to build confidence in their abilities to learn science. I want students to understand that I’m not trying to find out how smart they are.
I’m there to help them learn useful skills, and in doing so, appreciate that science is not just for the nerds in the class. They also need to appreciate that at some point we’re all going to be asked to vote on whether to allocate funds for science education and research.
Which Came First?
In most high schools, biology is taught before chemistry, presumably because of the mathematics needed for chemistry. I believe chemistry should be taught before biology, because so much of biology is chemistry of the cell. While there is more math in chemistry than biology, the math needed for chemistry, and for science in general, can be reviewed in 2-3 weeks, before teaching the science curriculum. To accomplish this, I have prepared a free math curriculum that reviews the math needed for grammar school, middle school, and high school science.
Introducing Them to Science
Not all kids are curious about science, but what all kids do want to learn is how to become stronger, more attractive, more appealing, and more respected. We need to sell them on the fact that science will help them become better athletes, better chefs, better hairdressers, better everything.
They need to know that science practically guarantees a longer and healthier life, because they’ll be better judges of smoking, drinking, recreational drugs, and hard drugs. They’ll also appreciate why exercise and a healthy diet are touted by all health magazines.
Moreover, we need to start teaching chemistry much earlier than we do now. About 10 years ago I walked into nearby Glenallen Grammar School and asked the principal for permission to teach chemistry to the 3rd grade class. I was granted an opportunity to teach five sections of the 3rd grade for four weeks. Using a van der Graaf generator, I showed students what electrons are and how, by flowing onto their hair, the negative electrons flowing onto their caused strands of hair to repel each other. From that, I showed them how positive and negative charges attract each other. After that I repeatedly cut apart drops of water until we got the smallest possible bit of water that we ended up calling a “molecule” of water.
Using a magnetic panel to attach metal rings to represent atomic orbits, and magnetic dots to represent electrons, we constructed simple atoms. I explained how, by moving electrons around, atoms bond to each other to make a molecule. I then used an electrolysis apparatus to show them how we could break water molecules apart, back into hydrogen and oxygen. Using handouts of paper atoms and electrons, the kids confirmed their understanding of how hydrogen and oxygen atoms bond to each other to make molecules of water.
Learning science has the benefit of bringing each learner a richer, more fulfilling life. From a practical standpoint, the Next Generation Science Standards have brought to the forefront the need to make science education usable. The two ways to do this are to teach so that students can see what being said as it’s being said, and to begin science education at a much earlier age, beginning with the structure of the atom.
About the author
Sheldon Margulies is a retired board-certified neurologist who has taught over 2500 medical students and residents at the University of Alabama and University of Maryland. He also has a law degree from the University of Baltimore.
Submit a Comment











